Why Does Temperature Affect The Kc . If you decrease the concentration of c, the top of the k c expression gets smaller. For an endothermic reaction such as: Changes in temperature change the equilibrium constant kc. When qc>kc, why there're more. Temperature dependence is another matter. After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. That would change the value of k c. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. Why equilibrium constant (kc) decreases if temperature increases? K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. When temperature is the stress that affects a system at. Why does the position of equilibrium move as it does? Explain how temperature changes affect a system at equilibrium.
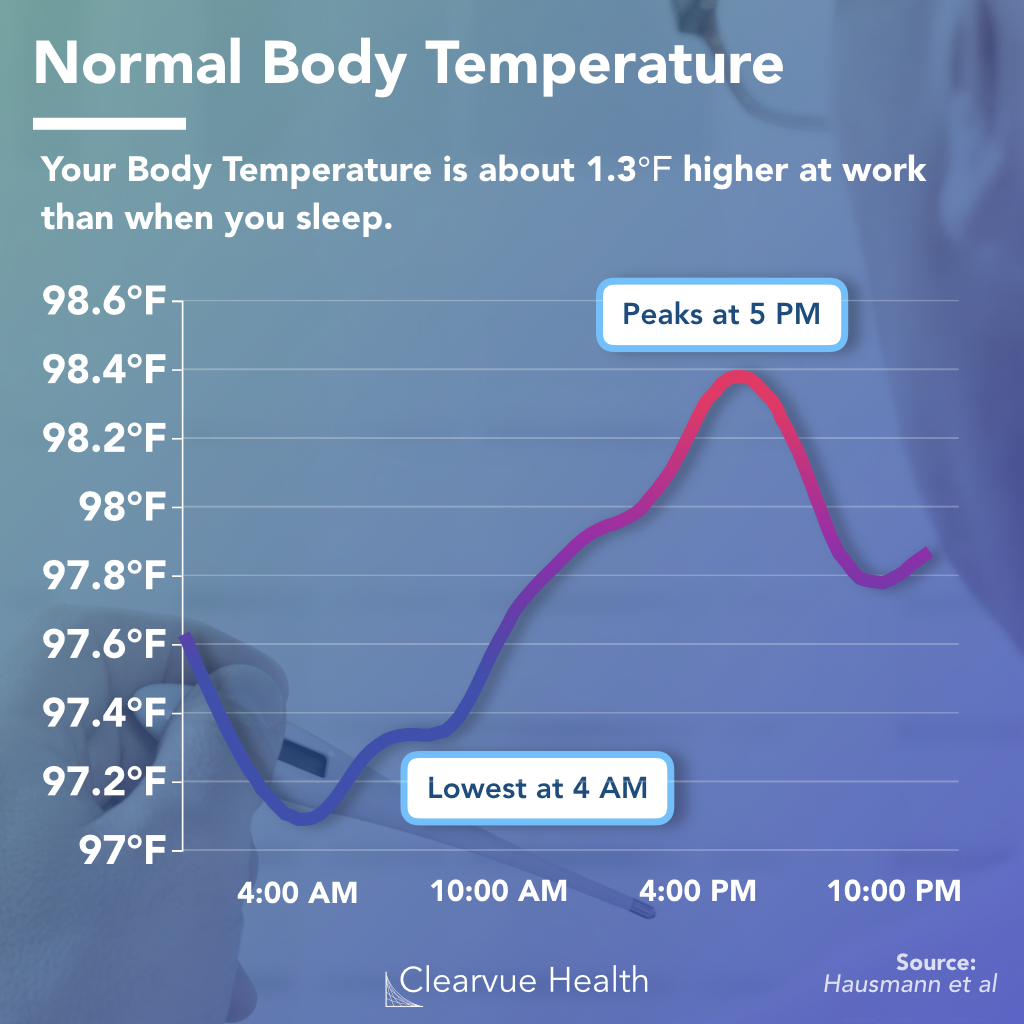
from www.clearvuehealth.com
After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. Why does the position of equilibrium move as it does? When qc>kc, why there're more. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. Changes in temperature change the equilibrium constant kc. Temperature dependence is another matter. For an endothermic reaction such as: Why equilibrium constant (kc) decreases if temperature increases? That would change the value of k c. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change.
The Redefinition of Human Body Temperature, and How It Affects Fever
Why Does Temperature Affect The Kc Temperature dependence is another matter. Temperature dependence is another matter. Explain how temperature changes affect a system at equilibrium. That would change the value of k c. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. Changes in temperature change the equilibrium constant kc. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. If you decrease the concentration of c, the top of the k c expression gets smaller. After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. Why equilibrium constant (kc) decreases if temperature increases? When temperature is the stress that affects a system at. When qc>kc, why there're more. Why does the position of equilibrium move as it does? For an endothermic reaction such as:
From www.researchgate.net
Diagrammatic illustration of body temperature in the human body. a In Why Does Temperature Affect The Kc Explain how temperature changes affect a system at equilibrium. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. Temperature dependence is another matter. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. Changes in temperature change. Why Does Temperature Affect The Kc.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Does Temperature Affect The Kc After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. When temperature is the stress that affects a system at. When qc>kc, why there're more. Explain how temperature changes affect a system at equilibrium. For an endothermic reaction such as: Why equilibrium constant (kc) decreases. Why Does Temperature Affect The Kc.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube Why Does Temperature Affect The Kc After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. When temperature is the stress that affects a system at. When qc>kc, why there're more. If you decrease the concentration of c, the top of the k c expression gets smaller. Why does the position. Why Does Temperature Affect The Kc.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Why Does Temperature Affect The Kc That would change the value of k c. When temperature is the stress that affects a system at. Changes in temperature change the equilibrium constant kc. Temperature dependence is another matter. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. K c values are only for an equilibrium at a specific temperature, if the temperature of. Why Does Temperature Affect The Kc.
From twitter.com
NWS Kansas City on Twitter "Today is the 3rd Thursday of the month Why Does Temperature Affect The Kc Why equilibrium constant (kc) decreases if temperature increases? Why does the position of equilibrium move as it does? Temperature dependence is another matter. Changes in temperature change the equilibrium constant kc. When temperature is the stress that affects a system at. Explain how temperature changes affect a system at equilibrium. That would change the value of k c. For an. Why Does Temperature Affect The Kc.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material Why Does Temperature Affect The Kc That would change the value of k c. For an endothermic reaction such as: When qc>kc, why there're more. Why equilibrium constant (kc) decreases if temperature increases? Explain how temperature changes affect a system at equilibrium. Why does the position of equilibrium move as it does? When temperature is the stress that affects a system at. After asking around i. Why Does Temperature Affect The Kc.
From www.numerade.com
SOLVED predict the change in the equilibrium constant KC that would Why Does Temperature Affect The Kc Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. Temperature dependence is another matter. Why does the position of equilibrium move as it does? Explain how temperature changes affect a system at equilibrium. When qc>kc, why there're more. For an endothermic reaction such as: K c values are only for an equilibrium at a specific temperature,. Why Does Temperature Affect The Kc.
From fitnessdriven.net
Why Is Kc Affected By Temperature? The 12 Top Answers Why Does Temperature Affect The Kc After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. Explain how temperature changes affect a system at equilibrium. If you decrease the concentration of c, the top of the k c expression gets smaller. That would change the value of k c. When qc>kc,. Why Does Temperature Affect The Kc.
From www.clearvuehealth.com
The Redefinition of Human Body Temperature, and How It Affects Fever Why Does Temperature Affect The Kc For an endothermic reaction such as: That would change the value of k c. Why does the position of equilibrium move as it does? When temperature is the stress that affects a system at. Explain how temperature changes affect a system at equilibrium. After asking around i have come to the conclusion that a reaction has many kc values depending. Why Does Temperature Affect The Kc.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Why Does Temperature Affect The Kc Explain how temperature changes affect a system at equilibrium. Changes in temperature change the equilibrium constant kc. Temperature dependence is another matter. That would change the value of k c. Why equilibrium constant (kc) decreases if temperature increases? Why does the position of equilibrium move as it does? When temperature is the stress that affects a system at. If you. Why Does Temperature Affect The Kc.
From www.youtube.com
Why does the temperature remain constant during a phase transition Why Does Temperature Affect The Kc Why equilibrium constant (kc) decreases if temperature increases? K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. Changes in temperature change the equilibrium constant kc. That would change the value of k c. Explain how temperature changes affect a system. Why Does Temperature Affect The Kc.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Why Does Temperature Affect The Kc Changes in temperature change the equilibrium constant kc. When qc>kc, why there're more. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. When temperature is the stress. Why Does Temperature Affect The Kc.
From www.chegg.com
Solved 2 2100 2HIg)2) Calculate Kc at this temperature for Why Does Temperature Affect The Kc Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. If you decrease the concentration of c, the top of the k c expression gets smaller. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change. Changes in. Why Does Temperature Affect The Kc.
From slideplayer.com
Aim How does temperature affect the solubility of solids, liquids, and Why Does Temperature Affect The Kc Why equilibrium constant (kc) decreases if temperature increases? When qc>kc, why there're more. When temperature is the stress that affects a system at. For an endothermic reaction such as: That would change the value of k c. After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. Why Does Temperature Affect The Kc.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Why Does Temperature Affect The Kc That would change the value of k c. When temperature is the stress that affects a system at. For an endothermic reaction such as: If you decrease the concentration of c, the top of the k c expression gets smaller. Why does the position of equilibrium move as it does? Temperature dependence is another matter. Changes in temperature change the. Why Does Temperature Affect The Kc.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1542354 Why Does Temperature Affect The Kc When qc>kc, why there're more. Why equilibrium constant (kc) decreases if temperature increases? Why does the position of equilibrium move as it does? Explain how temperature changes affect a system at equilibrium. Temperature dependence is another matter. Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. Changes in temperature change the equilibrium constant kc. When temperature. Why Does Temperature Affect The Kc.
From www.vrogue.co
How Does Temperature Affect Chemical Reaction vrogue.co Why Does Temperature Affect The Kc For an endothermic reaction such as: When temperature is the stress that affects a system at. Temperature dependence is another matter. After asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i don't see. K c values are only for an equilibrium at a specific temperature, if the. Why Does Temperature Affect The Kc.
From haipernews.com
How To Calculate Equilibrium Constant At Different Temperatures Haiper Why Does Temperature Affect The Kc Changes in temperature change the equilibrium constant kc. Why does the position of equilibrium move as it does? Because the value of \(\delta g_{rxm}^o\) is dependent on temperature, the value of. K c values are only for an equilibrium at a specific temperature, if the temperature of an equilibrium system is changed, the value of k c will also change.. Why Does Temperature Affect The Kc.